July 25, 2025 Learning How to Learn
Learning is important. And there is science around how to learn successfully. Quite an amount of science which is why I appreciate the course by Terry Sejnowski and Barbara Oakley titled Learning How to Learn [1]. It condenses a lot of science into practical advice.
As a submission for the final project of the course, I've written a small intro to an unrelated topic with bits of the information on how to learn interleaved. That unrelated topic is the basics of naming organic compounds according to the rules of the International Union of Pure and Applied Chemistry (IUPAC). I did my best to verify the content. Nevertheless I cannot guarantee correctness, I am by no means a chemist.
How to Learn New Things
In this "micro course" about the IUPAC nomenclature of organic chemistry, you will learn something about how names are given to various organic chemical compounds based on their structure. Why should you want to learn this? Perhaps because you will learn something about learning in the process as we will touch on some of the concepts of the Learning How to Learn course - but also because you can learn everything, whether it is related to things you already know or something entirely new, and learning will improve your life. Develop a growth mindset!
So, are you well rested? (Good sleep is in fact one of the important factors for success in learning. Another one is adequate exercise.)
Before we start, please consider using one practical, research-proven technique for learning, the Pomodoro technique: Eliminate distractions as best you can and set a timer for 25 minutes in which you focus intently on studying. When the alarm rings, stop what you're doing and take a five minute break. Repeat until you're done with the task or need to be somewhere else (sports, sleep, television, decide upfront how much time you want to spend with this). You may not need a full 25 minute Pomodoro for this.
Organic Chemistry - Carbon Bonds & Alkanes
Carbon bonding is essential for all known forms of life [2]. This is because of carbon's potential for different kinds of chemical bonds. Organic chemistry is the study of organic compounds, which are defined by some chemical authorities to be compounds that contain carbon-hydrogen or carbon-carbon bonds [3].
Because the field of chemistry is not exactly young, different compounds can have multiple common names, e.g., dichloromethane is the same as methylene chloride [4]. The International Union of Pure and Applied Chemistry (IUPAC) maintains a systematic naming scheme for organic compounds.
To systematically name an organic compound, biochemists first identify the parent chain. For the simple alkanes we're looking at now, this is the longest chain of carbon atoms. Its length and the types of its bonds determine the root name.
Take for example methane, ethane, propane, and butane:
| Methane | Ethane | Propane | Butane |
|---|---|---|---|

|

|

|

|
These are alkanes (all end in -ane). Alkanes consist of only carbon and hydrogen atoms and have carbon chains with only single bonds without forming cycles. Carbon has four electrons to form bonds, a single bond between two carbon atoms is formed by one electron of each carbon. Compared to structures with double or triple bonds, this leaves the most amount of possible other bonds. Alkanes and the carbons within them therefore called saturated as they bond to as many hydrogens as possible.
Before "ane" in methane, ethane, propane, and butane are "meth", "eth", "prop", and "but". These occur in other many other names too such as ethene or propyne. They signify, you guessed it, the length of the carbon chain. Meth- for one, eth- for two, prop for three, and but- for four carbon atoms in the longest carbon chain of a molecule. The mnemonic "Monkeys eat peeled bananas!" has helped a great many chemistry students to remember them.
Test!
A test? Already?
The most important thing to do while learning is to test oneself. Mini-tests throughout your learning sessions are a much more effective use of your time that re-reading the material several times [5]. Testing furthermore helps against developing the illusion of competence: When simply reading or watching material, it is very easy to think you understood the concepts as soon as you are able to follow the reasoning steps. Trying to recall the key points or (even better) solve exercise problems may show you that you in fact don't yet have a good understanding.
Question 1
- can be connected with single or double bonds.
- are each connected to exactly one hydrogen atom.
- form only single bonds to hydrogen and other carbon atoms.
Show Answer
Correct aswer: c.
Question 2
- eth (1 carbon), meth (2 carbons), but (3 carbons), prop (4 carbons)
- meth (1 carbon), eth (2 carbons), prop (3 carbons), but (4 carbons)
- prop (0 carbons), but (1 carbon), meth (2 carbons), eth (3 carbons)
Show Answer
Correct aswer: b.
Organic Chemistry - Alkenes, Alkynes
Beside the group of the alkanes with their single bonds and saturated carbons, there are other interesting groups. Let's have a look at alkenes and alkynes.
Ethene is an alkene. It is very similar to ethane, yet: It is an alkene, hence it ends on -ene. Two electrons are shared between the carbons, forming a double bond. Only two instead of three electrons are left to be shared with hydrogens to get a complete outer shell of eight electrons.
Alkanes have only single bonds, alkenes have at least one double bond, and alkynes, you guessed it, have at least one triple bond between carbon atoms. To determine what kinds of bonds exist in a carbohydrate, you can look at its base name:
- -ane: Only single bonds
- -ene: One or more double bonds, no triple bonds
- -yne: One or more triple bonds
In molecules with multiple carbon chains, double and triple bonds influence the identification of the parent chain. The parent chain becomes the longest out of those chains with the highest number of double and triple bonds. Tie-breaking rules apply if this is not unique. We don't need to worry about this for our simple examples now.
For now, let's check out some more alkenes:
(Do not worry about some of the hydrogens in But-1-ene being bundled in sub-molecules CH2 / CH3. This is just to keep it compact.)
Why are there numbers in the names? The fragment "ene" tells us that there is at least one double bond. It does not tell exactly how many and in which positions. The numbers tell us exactly that: The double bond of but-1-ene is in the first position of the longest carbon chain, and the double bonds of buta-1,3-diene are in positions one and three. The multiplicative prefix "di" gives another hint at the presence of two double bonds.
Note, that the structural formula of but-1-ene could have been written the other way around with the double bond on the right side and it would still be called but-1-ene. There are systematic rules about which end of the carbon chain to start counting from. Roughly speaking, we want to start counting from the end nearest to the most interesting feature of a molecule, keeping the numbers low.
Conceptualizing
For a learner to "get a grasp" on something, it is often helpful to jump back and forth between different sections in their study material. Before you start intensely memorizing specific aspects, say, the base name fragments for carbon chains of specific lengths, see if you have a feeling why they are useful and how they relate to other aspects, e.g., the bond type fragments. Start forming a mental concept by scanning over the sections and focusing on select aspects at a time. Your brain has the ability to switch between focused and diffuse modes of thinking to help you with that [1].
Test: Bond Types
- -ane, -ene, -yne
- -ene, -yne, -ane
- -ene, -ane, -yne
Show Answer
Correct answer: a.
-ane indicates molecules with only single bonds (saturated carbons)-ene indicates molecules with at least one double bond
-yne indicates molecules with at least one triple bond
Test: Name Some Molecules

- But-3-yne
- Butyne
- But-1-yne
Show Answer
Correct answer: c.
But-1-yne is a carbon chain of length 4 with one triple bond. Since a triple bond in a chain of 4 carbons can be either in the middle or with one of the outer carbons, we must specify the positions, and since there are no other interesting features, we begin counting at the side with the triple bond, placing it at position 1.

- Prop-2-yne
- Ethyne
- Propyne
Show Answer
Correct answer: c.
Propyne is an alkyne with one triple bond in the carbon chain. Because it does not matter which of the two bonds is the triple bond, we don't need to give the position in the name.
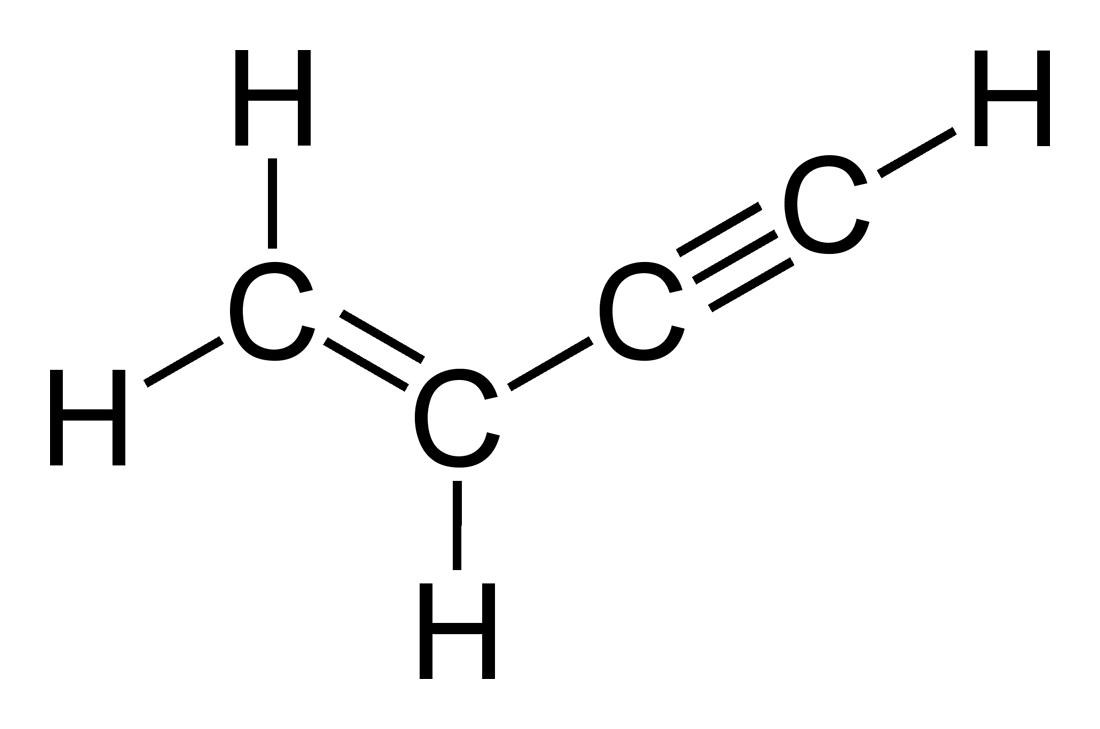
No given options. Note down your answer and check.
Hint
The chain is numbered from the end that assigns the lowest number to a double or triple bond. If multiple ends give the same number, the double bond is preferred.Show Answer
Correct answer: But-1-en-3-yne
Organic Chemistry - Substituents and Skeletal Formula
So far we've seen only molecules with a single chain of carbon atoms and enough hydrogen atoms to engage their remaining bonding sites. These are, of course, only the simplest of organic molecules.
Let's have a look at some more complex molecules and figure out how to name them systematically. Imagine a fully saturated carbon chain of length three (our friend Propane), except with one of the two hydrogens on the middle carbon substituted by another carbon. We want our molecule to be chemically stable, so attach three new hydrogens to the new carbon. The newly attached atoms (CH3) are called a methyl group1. They form a substituent in the carbon chain, and we get 2-Methylpropane: We identify the longest carbon chain and determine that we have a propane as our parent structure. The identification of substituents has lower seniority (read: priority), hence is done after the identification of the parent chain. Note: Lower seniority items typically produce fragments that appear earlier in the name, often as prefixes. Our methyl group at position two produces the prefix "2-Methyl".
Substituents don't have to be carbon-based. When a halogen atom like chlorine replaces a hydrogen, we get a haloalkane. In the case of Chloromethane, a chlorine atom has been substituted for one of methane's hydrogens. The prefix for a chlorine substituent is simply "chloro". Since methane has only one carbon atom, we don't need a number to indicate the substituent's position.
Structural formulas quickly get unwieldy (especially if all hydrogens are fully drawn out). A more concise alternative is the skeletal formula. Carbon atoms are represented by vertices and line-ends instead of the symbol C. Hydrogens bonded to carbons are omitted; it's assumed each carbon has enough to form four bonds. All other atoms are explicitly drawn with their chemical symbols. Double and triple bonds are shown as two and three lines respectively.
| But-1-ene | Buta-1,3-diene | Propyne | 2-Methylpropane | Chloromethane | |
| Structural Formula | |||||
| Skeletal Formula |
Chunking
As you practice, you are building chunks of knowledge. A chunk is a mental block of information that is connected through meaning. When you first see a skeletal formula, it might look like a random collection of lines. But with practice, you start to see it as a whole molecule, like 2-Chloropropane. You no longer need to count every carbon or hydrogen. This chunking process makes your thinking more efficient. It is one of the key ideas in learning anything new effectively.
You can create chunks by:
- Focusing your attention on the information you want to chunk.
- Understanding the basic idea. You can do this by seeing the connections between the parts.
- Practicing to gain context and see when to use the chunk.
Let's do some more tests to help solidify these chunks.
Test: Name Some Molecules

- Butane
- Pentane
- Hexane
Show Answer
Correct answer: b.
Each unlabled end of a line segment in the skeletal formula represents a carbon atom. Counting them, we find 5 carbons. Since there are only single bonds, it is an alkane. The name for a 5-carbon alkane is Pentane.

- But-1-ene
- Butane
- But-2-ene
Show Answer
Correct answer: a.
There are 4 carbon atoms and one double bond. We start counting from the end closest to the double bond, which places it at position 1.

- 1-Chloropropane
- 2-Chloropropane
- Trichloromethane
Show Answer
Correct answer: b.
The parent chain has 3 carbon atoms (Propane). A chlorine atom is attached to the second carbon atom.

- 1-Chloroprop-2-ene
- 3-Chloropropane
- 3-Chloroprop-1-ene
Show Answer
Correct answer: c.
The parent chain has three carbons and one double bond. We number from the right to give the double bond the lowest number (1), making it prop-1-ene. The chlorine atom is on carbon 3.
Functional Groups
A key concept that we have not yet looked at is the functional group, a specific group of atoms that largely determines the molecule's chemical properties.
Functional groups have higher seniority than substituents and bond types and different functional groups have different priority among themselves. The highest priority functional group is called the principal functional group. When present, the principal functional group often dictates the suffix of the molecule's name and gets priority in numbering the carbon chain. For example, when a hydroxyl (-OH) group and no higher-priority functional group is present, we speak of the molecule as an alcohol. This leads to one extra rule for identifying the parent chain: The parent chain is identified as the logest chain of carbons that conains the principal functional group.
For example, ethane with an -OH group becomes the alcohol Ethanol. The "-e" from "ane" is dropped and "-ol" is added. The hydroxyl group has priority over double/triple bonds for numbering.
This is just a glimpse. There are many functional groups (ketones, aldehydes, amines, etc.), each with its own suffix and priority rules, which is beyond the scope of this introduction.
Let's recap the general approach to IUPAC naming we've seen:
- Identify the Parent Structure: Find the longest continuous carbon chain that contains the principal functional group or main unsaturation (double/triple bonds). This gives the base name (e.g., propane, butane).
- Identify Principal Features: Principal features like double bonds (-ene), triple bonds (-yne), and principal functional groups (e.g., -ol) determine the suffix and have priority in numbering.
- Number the Chain: Number the carbons in the parent chain to give the lowest possible numbers to principal features, starting with the highest priority group.
- Name Substituents: Identify any side groups (e.g., methyl-) or other atoms (e.g., chloro-) and give them a position number.
- Assemble the Full Name: Combine the pieces, often in the form (position)-(substituent) (base name)(position)-(bond type suffix) (position)-(functional group suffix).
The End
I hope you learned something about learning and/or have become interested in the Learning how to Learn course (or in biochemistry). Perhaps you're currently taking the course, in which case I'd be especially interested in how useful you found this practical example of something to learn and whether you think that the usefulness of the learning techniques have proven useful. My intention was to provide some example not necessarily within everybody's daily life, interleaved with bits from Learning how to Learn, to demonstrate that
- you can learn anything you want,
- anything you learn broadens your perspective and concepts can be transferred to other areas, and
- training how to learn is helpful, especially practice and repetition over cramming.
One More Test
Let's try to name one more molecule with a functional group, this time an aldeyde. The aldehyde functional group contains a carbon atom double-bonded to an oxygen atom and single-bonded to a hydrogen atom. This leaves only one electron to bond to anything else, hence it is always located at the end of a carbon chain. Aldehydes get the suffix -al.

Fill in the blanks: _-methyl____al
Show Answer
Correct answer: 3-methylbutanal.
The parent chain has 4 carbons (butane) and an aldehyde group (-al). The aldehyde carbon is always at position 1, placing the methyl group at position 3.